Which Gas Causes Acid Rain
Due to which gas is acid rain caused. 5 min read.
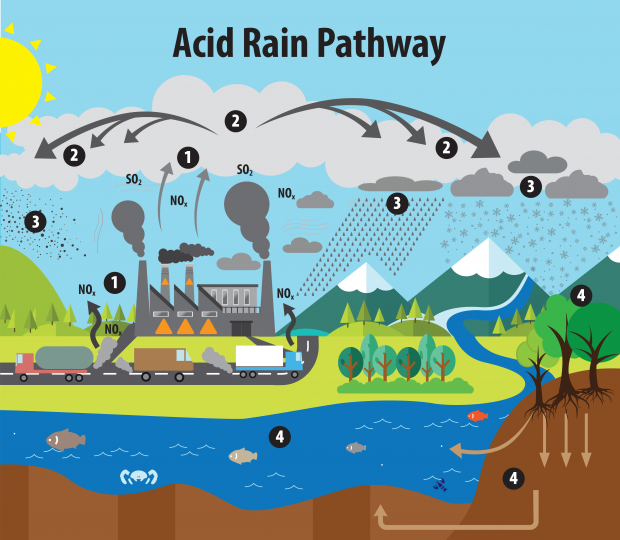
How Air Pollution Causes Acid Rain Breeze Technologies
Volcanic acid rain pH 25-50 is typically dominated by hydrochloric acid HCl and sulfuric acid H 2 SO 4 arising from the plume gases HCl and sulfur dioxide SO 2.

. CO 2 H 2 O H 2 CO 3. Which pollution causes acid rain 1. SO2 and NO2 3.
What causes acid rain. Published February 28 2019. The gas most responsible for the acid rain effect on plants and water systems is sulfur dioxide.
Yes acid rain is still around and yes its still a problem. Common Questions and Answers about What gas causes acid rain. The normal rain is slightly acidic having a pH of about 56 as carbon dioxide gas reacts with it to form a weak carbonic acid.
But acid rain can have a pH of about 50-55 and can even be in the 4 range in. Out of gas is what i feel likei take 80mg. View solution What is an estimated damage caused by acid rain to buildings in USA.
Most water including drinking water has a neutral. Up to 24 cash back Acid Rain is a form of precipitation that is unusually acidic because of atmospheric pollution from sulfur and nitrogen oxides. Sulfurdioxide SO2 is a gas that is converted into Sulfuric acid.
It can also occur in the form. Acid rain leads to a change in these living conditions since it makes the soil more acid. Two gases are the main cause of acidic rain.
A higher level of acidity in the soil leads to a change in the growth behavior of plants. Acid rain results when sulfur dioxide SO 2 and nitrogen oxides NO X are emitted into the atmosphere and transported by wind and air currentsThe SO 2 and NO X react with. Of oxycontin at 8a 2p and 10pmi dont get high.
Acid rain is formed by elevated levels of sulfur and nitric acids in the atmospheres that accumulate as a result of Nitrogen oxides NOx and Sulfur dioxides SO2. Rain is naturally slightly acidic since picks up carbon dioxide in the air producing carbonic acid. Prev Question Next Question.
The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rainSulphur and Nitrogen particles which get mixed with water are. Normal clean rain has a pH between 5 -. What gas is dissolved in acid rain that causes it to be acidic.
Power plants release the majority of sulfur dioxide and much of the nitrogen oxides when they burn fossil fuels such as coal to produce electricity. Primary Causes of Acid Rain. The process of acid.
Sulfur dioxide and nitrogen oxides react with water oxygen and oxidants and forms. In addition the exhaust from cars trucks. The main chemicals in air pollution that create acid rain are sulfur dioxide SO2 and nitrogen NO2.
The world is changing for the worse. What gas causes acid rain. Causes of Acid Rain.
Pure water has a pH of 7 and generally rainfall is somewhat on the acidic side a bit less than 6. The main acid rain. Acid rain is rain or any other form of precipitation that is unusually acidic meaning that it has elevated levels of hydrogen ions low pH.
Several gases resulting from fossil fuel combustion can form acidic compounds. Acid rain caused by burning coal and oil products to fuel our societys needs has damaged trees in many areas of North America. View solution View more.
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids.
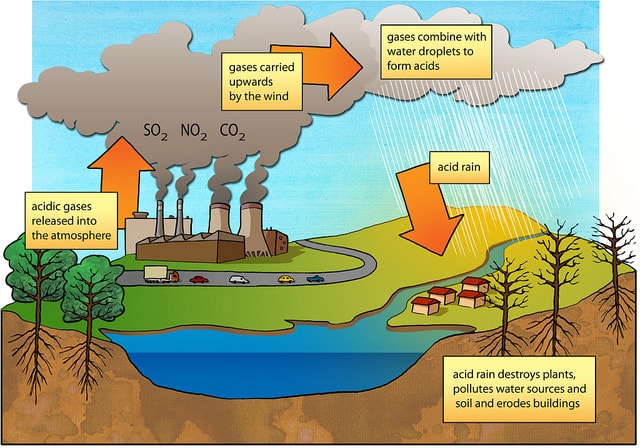
Primary Causes Of Acid Rain Earth Eclipse
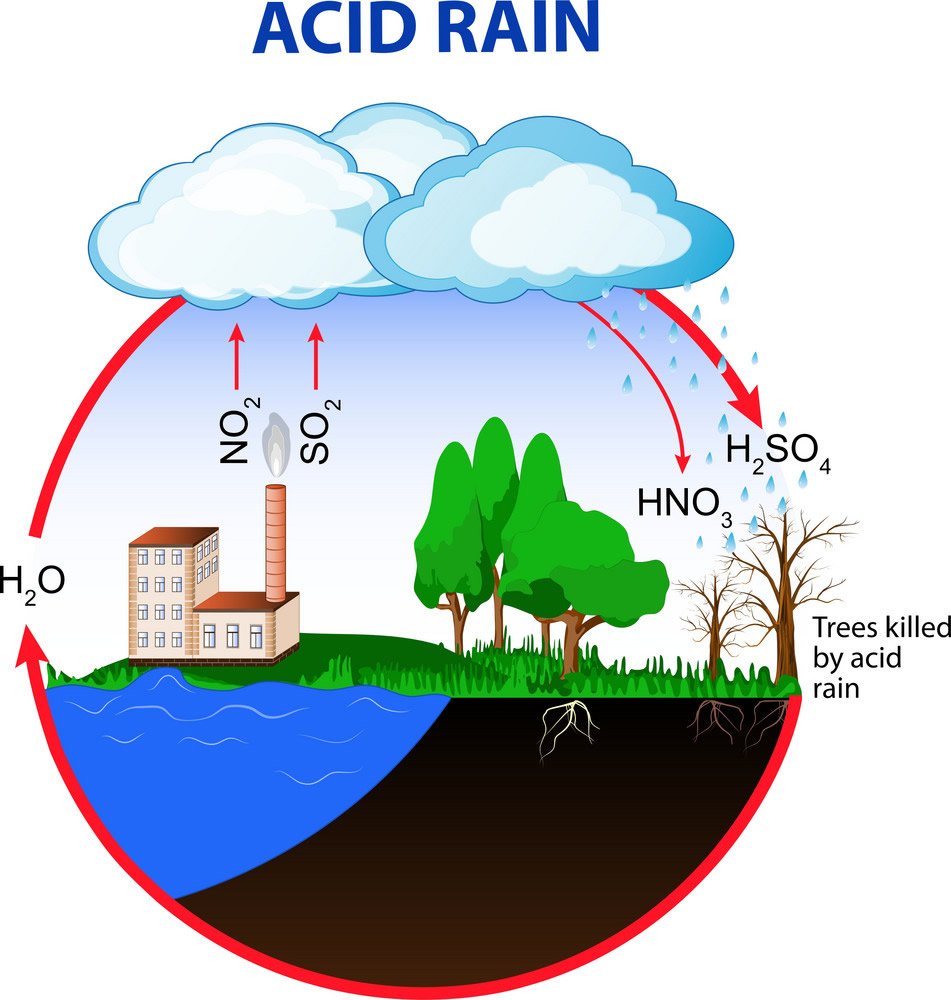
What Is Acid Rain Internet Geography
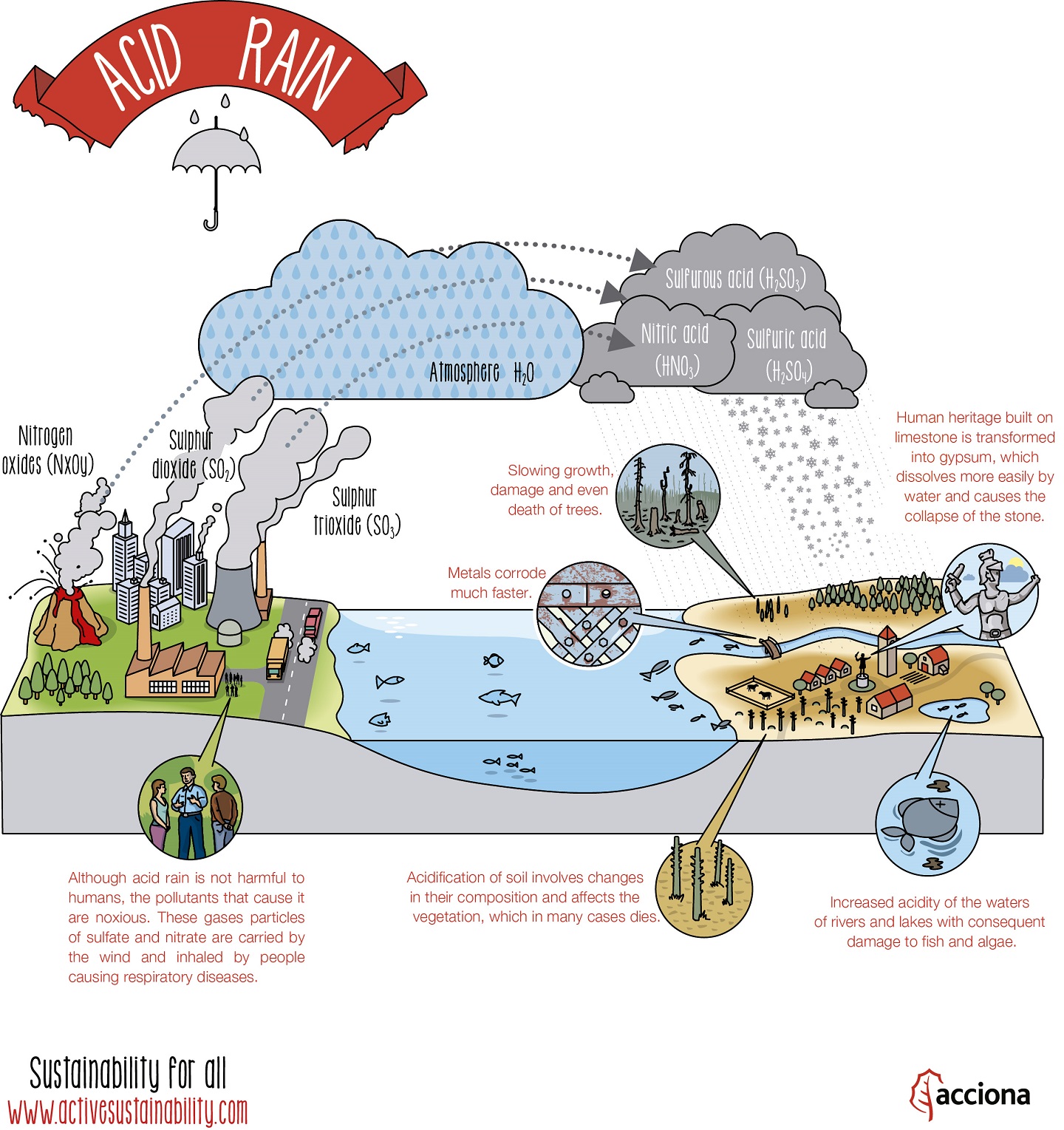
What Is Acid Rain And How Is It Formed

Thumbnail For Version As Of 16 58 30 August 2008 Pemanasan Global Hujan Gambar
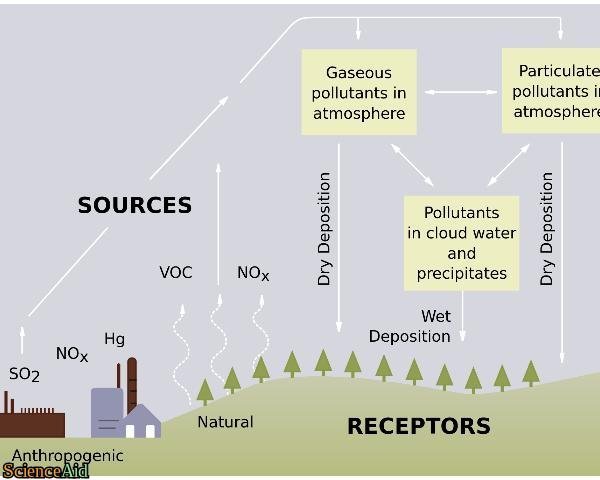

Comments
Post a Comment